[导读] 来源:医学界肿瘤频道
PD-L1(SP363)检测上市助力免疫治疗诊断新时代。
今年5月,继美国食品药品监督管理局(FDA)、欧盟CE IVD批准后,罗氏诊断VENTANA PD-L1(SP263)免疫组织化学法(IHC)伴随诊断获国家药品监督管理局(NMPA)批准上市[1]。

“随着精准治疗不断进展,继靶向治疗后,近年来癌症免疫治疗不但在国外飞速发展,国内临床应用也是日新月异,对于一些单药或一线/二线治疗适应证获批越来越多。关于如何更好地遴选合适患者、预测疗效,目前最有效和应用最广泛的方法是肿瘤组织的PD-L1检测,PD-L1高表达患者更能从相应治疗中获益,因而PD-L1的精准评估对于甄选潜在治疗获益患者、尽可能避免低效或无效治疗的临床诊疗决策意义重大。”浙江大学医学院附属第一医院病理科滕晓东教授表示,“此次PD-L1(SP263)临床病理检测抗体被批准在国内上市,特别是作为国内第一个尿路上皮癌免疫治疗的伴随诊断产品,主要是基于国人数据的临床前瞻性研究获批的PD-L1检测,具有重要的临床意义和病理价值。首先祝贺PD-L1(SP263)上市,也期待PD-L1(SP263)检测能更好地服务临床与患者。”
PD-L1 IHC是评估PD-(L)1抑制剂疗效的唯一方法学[2,3]
近年来,随着肿瘤免疫检查点生物学机制研究进展,PD-1/PD-L1免疫检查点抑制剂在癌症治疗中取得突破性进展,正迅速改变着多种癌症的治疗模式。截至发稿日,NMPA已批准8款、美国FDA已批准6款PD-1/PD-L1抑制剂用于临床治疗多种癌症类型[4]。
基于PD-1/PD-L1抑制剂的免疫治疗正深刻改变着多种癌症治疗范式和临床诊治实践。然而,如果不加分层选择地使用PD-1/PD-L1抑制剂于多种适应证,其整体有效率仍然偏低,大约徘徊在10%~30%之间[5-10](图1)。因此,通过合适的生物标志物预测评估患者从PD-1/PD-L1抑制剂治疗中潜在获益的情况,对提升临床整体有效率意义重大。
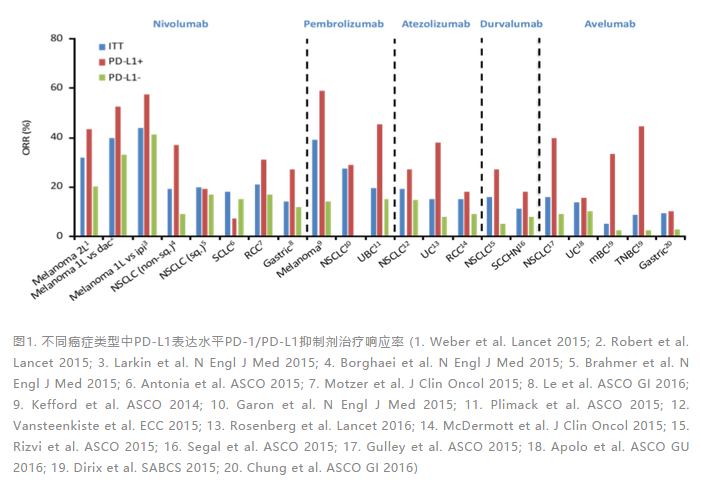
多项前瞻性临床研究结果,包括NCT01693562[11]、Pacific[12,13]、IMpassion130[14]、KEYNOTE-024[15]、CheckMate057[16]以及此次PD-L1(SP263) NMPA获批BGB-A317-204[17]等,均已充分证明在多种癌症类型相应适应证进行PD-1/PD-L1抑制剂治疗时,通过评估标志物PD-L1表达进行分层,PD-L1高表达往往意味着患者更能从PD-1/PD-L1免疫检查点抑制剂治疗中获益[2,3]。
PD-L1表达水平评估是目前临床证据支持最充分、临床实践最广泛的PD-1/PD-L1抑制剂疗效预测标志物。而PD-L1表达评估只能通过蛋白层面检测,因此IHC是目前临床诊断级别评估肿瘤组织PD-L1表达水平的唯一方法学[2,3,18]。
PD-L1(SP263)检测上市助力免疫治疗诊断新时代
截至发稿日,国内已有预期用于非小细胞肺癌、小细胞肺癌、尿路上皮癌等多种适应证的PD-1/PD-L1抑制剂获批,或作为治疗决策先决条件,临床实践中对PD-L1 IHC伴随诊断的需求亟待满足。此次PD-L1(SP263) IHC检测获批成为国内首个预期用于尿路上皮癌PD-1抑制剂治疗决策伴随诊断,对于提升临床实践中相应免疫治疗的响应率和整体获益价值巨大[17,19]。随着该检测后续更多适应证的获批,将为更多癌症患者带来福祉。
根据获批公布信息可知,VENTANA PD-L1 (SP263) IHC伴随诊断临床注册获批检测体系(图2)基于BenchMark ULTRA全自动免疫组化染色系统,预期用于评估BenchMark ULTRA运行、DAB染色液(OptiView DAB IHC Detection Kit)显色的福尔马林固定、石蜡包埋(FFEP)尿路上皮癌肿瘤组织中的PD-L1表达状态,用于辅助评估相关患者是否能从相关免疫治疗获益[17,20]。
尿路上皮癌现有化疗有效率有限,而免疫治疗近年在临床实践中的发展使化疗后进展或转移患者有了新的治疗机会,特别对于肿瘤组织PD-L1高表达患者,往往能有更高获益[30]。
临床病理实践中,PD-L1表达评估主要基于肿瘤组织标本上进行IHC检测。一方面,IHC属于病理科常规开展成熟项目;另外一方面,PD-L1的表达不同于传统靶向治疗相关标志物有或无的特征,往往具有连续性和不同部位可能存在异质性的特征。因此,对于国内PD-L1 IHC检测在病理实验室的标准化、规范化开展仍需考虑不同层面的评估和论证,以保证PD-L1检测标准的统一和结果的准确,这包括抗体和检测体系的选择、实验室质控的评估以及判读共识的明确与统一等[21,22]。
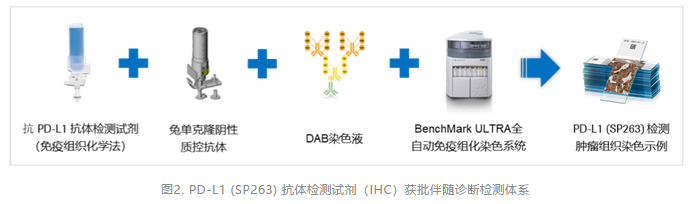
PD-L1(SP263)检测上市或利于PD-L1 IHC检测规范化、标准化[21,22]
作为临床治疗方案决策诊断级检测,经NMPA和/或FDA和/或CE IVD认证的PD-L1 IHC检测体系已有4种,均与特定药物和特定判读方法相关,不同PD-L1 IHC检测体系的临床治疗疗效预测价值在相应研究中亦得到相应临床验证[3,21-23]。4种获批PD-L1 IHC检测体系均高度标准化、即用型检测试剂盒,除不同特定一抗(克隆号),均使用不同检测二抗系统、软件方案和平台。其中,VENTANA PD-L1(SP263)检测和VENTANA PD-L1(SP142)检测适用于BenchMark全自动化平台,Dako PD-L1(22C3) pharmDx和Dako PD-L1(28-8) pharmDx适用于AutoStainer Link 48平台[2,3,21,22]。
由于不同癌症类型之间肿瘤免疫微环境的差异[24,25],以及不同PD-1/PD-L1抑制剂相应临床注册研究分层PD-L1 IHC检测体系差异[18,21,26],导致往往不同的PD-1/PD-L1抑制剂对应不同的PD-L1 IHC检测体系、不同判读方法及阈值,给临床实践中病理实验室PD-L1检测体系的选择和PD-L1 IHC检测的规范化、标准化带来一定挑战[21]。
在基于法规批准和临床试验证据支持的前提下,不同检测体系之间的一致性数据仍在积累中。其中,IASLC PD-L1 IHC Blueprint (BP)I期和II期研究证实PD-L1(SP263)和PD-L1(22C3)、PD-L1(28-8)检测体系在评估肿瘤细胞(Tumor Cell, TC)染色性能方面整体一致性很高[27,28]。另外一项AstraZeneca一致性评价研究中,在多种临床诊治决策PD-L1表达评估阈值(1%、10%、25%和50% TPS)下,PD-L1(SP263)和PD-L1(22C3)、PD-L1 (28-8)检测体系之间的总体一致性百分比大于90% [21,29]。
“目前VENTANA PD-L1(SP263)检测已被CE IVD认证批准用于度伐利尤单抗、纳武利尤单抗和帕博利珠单抗三种药物相关适应证的检测[21-23],这对于PD-L1 IHC检测体系的标准化和规范化发展将有一定推动和促进作用。根据已披露的免疫检测点抑制剂相关临床研究进展,预期将会有更多的适应证获批,”滕晓东教授表示,“目前来看,在PD-L1 IHC临床实践中推进标准化、规范化建设仍任重道远。如针对CE IVD认证多种免疫检测点抑制剂适应证,PD-L1(SP263)检测体系能否在未来应用于更多试验中,以积累临床实践支持的证据用于更多药物及肿瘤的检测,以促进PD-L1 IHC检测标准化、规范化发展以及进一步趋向评判标准和检测抗体的统一或将值得期待。”
参考文献:
[1]http://app1.sfda.gov.cn/datasearchcnda/face3/base.jsp?tableId=27&tableName=TABLE27&title=%BD%F8%BF%DA%C6%F7%D0%B5&bcId=152904442584853439006654836900
[2]Tsao, MING SOUND, et al. IASLC atlas of PD-L1 immunohistochemistry testing in lung cancer. Aurora, CO: International Association for the Study of Lung Cancer (2017).
[3]2018 ESMO handbook of immune-oncology.
[4]www.cancerresearch.org/scientists/immuno-oncology-landscape/pd-1-pd-l1-landscape
[5]Aguiar PN Jr, De Mello RA, Hall P, Tadokoro H, Lima Lopes G. PD-L1 expression as a predictive biomarker in advanced non-small-cell lung cancer: updated survival data. Immunotherapy. 2017;9(6):499-506
[6]Daud AI, Wolchok JD, Robert C, et al. Programmed Death-Ligand 1 Expression and Response to the Anti-Programmed Death 1 Antibody Pembrolizumab in Melanoma. J Clin Oncol. 2016 Dec;34(34):4102-4109
[7]Chow LQ, Haddad R, Gupta S, et al. Antitumor Activity of Pembrolizumab in Biomarker-Unselected Patients With Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results From the Phase Ib KEYNOTE-012 Expansion Cohort. J Clin Oncol. 2016 Sep 19. pii: JCO681478.
[8]Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017 Jan
[9]Jiang, Youhai, et al. Progress and challenges in precise treatment of tumors with PD-1/PD-L1 blockade. Frontiers in Immunology 11 (2020).
[10]Hunter, Katerina Ancevski, Mark A. Socinski, and Liza C. Villaruz. PD-L1 testing in guiding patient selection for PD-1/PD-L1 inhibitor therapy in lung cancer. Molecular diagnosis & therapy 22.1 (2018): 1-10.
[11]Powles, Thomas, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA oncology 3.9 (2017): e172411-e172411.
[12]Antonia, Scott J., et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. New England Journal of Medicine 377.20 (2017): 1919-1929.
[13]Antonia, Scott J., et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. New England Journal of Medicine 379.24 (2018): 2342-2350.
[14]Schmid, Peter, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet Oncology 21.1 (2020): 44-59.
[15]Reck, Martin, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. New England Journal of Medicine 375.19 (2016): 1823-1833.
[16]Borghaei, Hossein, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. New England Journal of Medicine 373.17 (2015): 1627-1639.
[17]Ye, D., et al. First report of efficacy and safety from a phase II trial of tislelizumab, an anti-PD-1 antibody, for the treatment of PD-L1+ locally advanced or metastatic urothelial carcinoma (UC) in Asian patients. Annals of Oncology 30 (2019): v367.
[18]Davis, Andrew A., and Vaibhav G. Patel. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. Journal for immunotherapy of cancer 7.1 (2019): 278.
[19]https://news.bioon.com/article/6753711.html
[20]抗 PD-L1 (SP263) 抗体检测试剂(免疫组织化学法)说明书.
[21]Prince, Spasenija Savic, and Lukas Bubendorf. Predictive potential and need for standardization of PD-L1 immunohistochemistry. Virchows Archiv 474.4 (2019): 475-484.
[22]Lantuejoul, Sylvie, et al. PD-L1 Testing for Lung Cancer in 2019: Perspective From the IASLC Pathology Committee. Journal of Thoracic Oncology (2019).
[23]VENTANA PD-L1 (SP263) Assay (CE IVD) Package Insert.
[24]Hegde, Priti S., Vaios Karanikas, and Stefan Evers. The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. (2016): 1865-1874.
[25]Hegde, Priti S., and Daniel S. Chen. Top 10 Challenges in Cancer Immunotherapy. Immunity 52.1 (2020): 17-35.
[26]Hunter, Katerina Ancevski, Mark A. Socinski, and Liza C. Villaruz. PD-L1 testing in guiding patient selection for PD-1/PD-L1 inhibitor therapy in lung cancer. Molecular diagnosis & therapy 22.1 (2018): 1-10.
[27]Hirsch, Fred R., et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. Journal of Thoracic Oncology 12.2 (2017): 208-222.
[28]Tsao, Ming Sound, et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project. Journal of Thoracic Oncology 13.9 (2018): 1302-1311.
[29]Ratcliffe, Marianne J., et al. Agreement between programmed cell death ligand-1 diagnostic assays across multiple protein expression cutoffs in non–small cell lung cancer. Clinical Cancer Research 23.14 (2017): 3585-3591.
[30]Powles, Tom, and Laura Morrison. Biomarker challenges for immune checkpoint inhibitors in urothelial carcinoma. Nature Reviews Urology 15.10 (2018): 585-587.















共0条评论