Abstract: As in other organ systems, immunohistochemistry (IHC) serves as an ancillary diagnostic tool for a wide variety of neoplastic and non-neoplastic disorders, including infections, work-up of inflammatory conditions, and subtyping neoplasms of the gastrointestinal (GI) tract. In addition, IHC is also used to detect a variety of prognostic and predictive molecular biomarkers for carcinomas of the GI tract. The purpose of this review is to highlight the use of IHC in common diagnostic scenarios throughout the tubular GI tract. The clinical indication and guidelines for performing IHC for detecting Helicobacter pylori is discussed along with role of gastrin and neuroendocrine markers in the diagnosis of autoimmune metaplastic atrophic gastritis. The major portion of this review discusses the use of IHC in the diagnostic workup of malignant neoplasms of the GI tract, such as adenocarcinoma versus squamous cell carcinoma, workup of poorly differentiated malignant neoplasms, and evaluation of uncommon gastric neoplasms (alphafeto protein–producing carcinomas) and switch/sucrose nonfermenting complex-deficient carcinomas. Lastly, localization of neuroendocrine tumors of unknown origin to aid clinical management, as well as HPV-driven anal neoplasia and IHC in the workup of basaloid anal neoplasms are also reviewed. | 摘要:与其他器官系统一样,免疫组织化学(IHC)可作为多种肿瘤性和非肿瘤性疾病的辅助诊断工具,包括感染、炎症状况的调查和胃肠道肿瘤分类。此外,IHC还可用于检测胃肠道癌的多种预后和预测性分子生物标记物。本综述的目的是强调IHC在整个胃肠道常见诊断方案中的应用。探讨了IHC检测幽门螺杆菌的临床适应证和指导原则,以及胃泌素和神经内分泌标记物在自身免疫性化生性萎缩性胃炎诊断中的作用。本综述的主要部分讨论了IHC在胃肠道恶性肿瘤诊断中的应用,如腺癌与鳞状细胞癌,低分化恶性肿瘤的诊断,以及对罕见的胃肿瘤(产生α-甲胎蛋白的癌)和SWI/SNF复合物缺陷性癌的评估。最后,还对原发部位不明的神经内分泌肿瘤进行定位以帮助临床治疗,以及对HPV驱动的肛门肿瘤和IHC在基底样肛门肿瘤诊断中的作用进行了综述。 |
|
In the gastrointestinal (GI) tract, immunohistochemistry (IHC) serves as an ancillary diagnostic tool for a wide variety of neoplastic and non-neoplastic disorders, including infections [eg, Helicobacter pylori (HP), cytomegalovirus, herpes simplex virus], work-up of inflammatory conditions (autoimmune atrophic gastritis), and subtyping tumors. In addition, IHC is also used to detect a variety of prognostic and predictive molecular biomarkers (HER2, mismatch repair proteins, PD-L1) for carcinomas of the GI tract. The purpose of this review is to highlight the use of IHC in common diagnostic scenarios throughout the tubular GI tract. We will focus on topics which are most likely to be encountered by a surgical pathologist and those that have received recent attention in the literature in terms of recommendations for standardized clinical practice. | 在胃肠道(GI)中,免疫组织化学(IHC)可作为多种肿瘤性和非肿瘤性疾病的辅助诊断工具,包括感染[如幽门螺杆菌(HP)、巨细胞病毒、单纯疱疹病毒]、炎症状况的调查(自身免疫性萎缩性胃炎)和肿瘤亚型。此外,IHC还可用于检测胃肠道癌的多种预后和预测性分子生物标记物(HER2,错配修复蛋白,PD-L1)。本综述的目的是强调IHC在整个胃肠道常见诊断方案中的应用。我们将集中讨论外科病理医生最有可能遇到的话题,以及那些在文献中就标准化临床实践的建议而受到关注的话题。 |
IMMUNOMARKERS TO EVALUATE GASTRITIS | 评价胃炎的免疫标志物 |
The Sydney System classifies gastritis into 3 categories: chronic gastritis, acute gastritis, and a variety of special forms of gastritis. | 悉尼系统将胃炎分为三类: 慢性胃炎、急性胃炎和各种特殊类型的胃炎。 |
Chronic gastritis can be further classified based on the underlying etiology and the presence and pattern of atrophy. HP infection and autoimmune metaplastic atrophic gastritis (AMAG) are the most well-characterized etiologies of chronic gastritis. Both can result in gastric atrophy but affect different parts of the stomach. Chronic HP infection results in antral-predominant gastritis and multifocal atrophic gastritis with atrophy of both antrum and corpus, while AMAG diffusely affects the corpus, while sparing the antrum. Atrophy is the first step in the sequence of the development of gastric adenocarcinomas, and both multifocal HP-related atrophic gastritis and AMAG follow this sequence of carcinogenesis. Furthermore, HP infection is strongly associated with extranodal marginal zone lymphomas of the stomach, while AMAG predisposes to gastric neuroendocrine tumors (NET) and gastric adenomas. Determining the etiology of gastritis is therefore important, not only for clinical management, but also for prognosis. Fortunately, IHC can be used in a variety of ways to facilitate this process. | 慢性胃炎可以根据潜在的病因和萎缩的存在和类型进一步分类。HP感染和自身免疫性化生性萎缩性胃炎(AMAG)是慢性胃炎最典型的病因。两者均可导致胃萎缩,但影响胃的不同部位。慢性HP感染是以累及胃窦为主的胃炎和胃窦和胃体均萎缩的多灶性萎缩性胃炎,而AMAG广泛影响胃体,胃窦不受累。萎缩是胃腺癌发生的第一步,多灶性HP相关性萎缩性胃炎和AMAG均遵循这一癌变过程。此外,HP感染与胃结外边缘区淋巴瘤密切相关,而AMAG易发生胃神经内分泌肿瘤(NET)和胃腺瘤。因此,确定胃炎的病因不仅对临床治疗,而且对预后也很重要。幸运的是,通过IHC可以解决这一问题。 |
(一)Detection of Helicobacter pylori | (一)幽门螺旋杆菌的检测 |
IHC offers superior sensitivity for HP detection compared with traditional histochemical stains (eg, Giemsa, Warthin-Starry) and has replaced these stains in many laboratories(Table 1). | 与传统的组织化学染色(如Giemsa、Warthin-Starry)相比,IHC对HP的检测具有更高的灵敏度,并已在许多实验室中取代了这些染色(表1)。 |
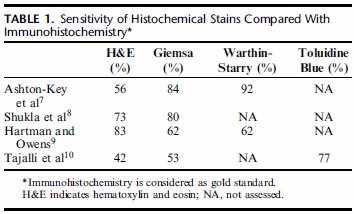
| 图1:组织化学染色与免疫组织化学的敏感性比较 免疫组织化学被认为是金标准。 NA是指没有评估。 |
Most cases of HP gastritis manifest as chronic gastritis with prominent neutrophilic activity, and thus, can be diagnosed on hematoxylin and eosin (H&E) stain alone. | HP胃炎多表现为中性粒细胞活性显著的慢性胃炎,仅苏木精-伊红染色即可确诊。 |
The key question therefore is: when is HP IHC indicated? Are there any histologic criteria that can be used to recognize cases with minimal inflammation as “HP negative” on the basis of H&E stain alone? | 因此,关键问题是:何时提示要做IHC检测HP?是否有任何组织学标准可用于在单纯H&E染色的基础上,将炎症程度最小的病例识别为“HP阴性”? |
The absence of organisms on routine H&E stain, especially in the presence of active gastritis or marked chronic inactive gastritis should prompt evaluation by IHC. | 常规H&E染色,尤其是在活动性胃炎或明显的慢性活动性胃炎存在的情况下,未见微生物时应及时做IHC来进行评估。 |
In these biopsies, paucity of organisms on H&E stain can be secondary to presence of intestinal metaplasia, erosions or ulcers, proton pump inhibitor use, sampling variability, recent antibiotic therapy, or Helicobacter heilmannii infection. | 在这些活检标本中,H&E染色未见微生物可能继发于肠化生、糜烂或溃疡、质子泵抑制剂的使用、取样变异、最近的抗生素治疗或海尔曼螺杆菌感染。 |
There is less agreement about performing IHC in cases with mild chronic inactive gastritis and histologically normal gastric mucosa. Several prospective studies that have explored the lower limit of gastric inflammatory activity which can potentially harbor HP organisms found that HP gastritis is usually accompanied by at least some degree of acute or chronic inflammatory activity. | 轻度慢性非活动性胃炎与组织学正常胃黏膜行IHC的一致性较差。一些前瞻性的研究发现,HP胃炎通常伴有一定程度的急性或慢性炎症活动,这些研究探索了胃炎症活动的下限,而胃炎症活动可能藏匿HP这种微生物。 |
Thus, employing HP IHC in histologically normal gastric mucosa is not indicated. | 因此,在组织学正常的胃黏膜中使用HP-IHC是没有意义的。 |
A large prospective study by Glickman et al challenged this conclusion. | Glickman等人的一项大型前瞻性研究对这一结论提出了质疑。 |
In this study, the authors evaluated biopsies from 81,648 patients and found that 9.3% (n=7664) cases were HP positive. Of the positive cases, only 0.2% (n=156) biopsies showed minimal or no inflammation. | 在这项研究中,作者评估了81648名患者的活检结果,发现9.3%的患者为HP阳性。阳性病例中,仅有0.2%的活检显示轻微或无炎症反应。 |
These patients showed similar demographics when compared with typical HP gastritis patients, but were more likely to have a history of prior HP infection. The authors concluded that in biopsies with minimal or no inflammation, infection is unlikely to be suspected based on histopathologic features, and a universal gastric biopsy staining protocol is the best way to avoid missing HP infection. | 与典型HP胃炎患者相比,这些患者显示出相似的人口统计学特征,但更有可能有HP感染史。作者的结论是,在组织病理学特征的基础上,很少或没有炎症的活检不太可能怀疑感染,通用的胃活检染色方案是避免遗漏HP感染的最佳方法。 |
Helicobacter pylori is almost never present in normal gastric mucosa. Therefore, it is not cost-effective to perform upfront HP stains without histological assessment of the biopsy. | 幽门螺杆菌几乎不存在于正常胃黏膜。因此,如果不进行活检的组织学评估就进行HP染色是不划算的。 |
Thus, per the Rodger Haggitt Gastrointestinal Pathology Society Consensus Guidelines, “upfront” HP IHC is no longer recommended, and ancillary stains are considered appropriate only when biopsies show chronic, or chronic active, gastritis without detectable HP in H&E-stained sections. | 因此,根据Rodger-Haggitt胃肠病理学会共识指南,不再推荐“预先”HP IHC,仅当活检显示慢性或慢性活动性胃炎,H&E染色切片中未检测到HP时,才认为辅助染色是合适的。 |
Application of these guidelines relies on accurate diagnosis of chronic gastritis. Unfortunately, the minimal threshold for defining chronic gastritis has not been standardized. | 这些指南的应用依赖于慢性胃炎的准确诊断。不幸的是,定义慢性胃炎的最低限度还没有标准化。 |
A commonly used criterion adapted from the updated Sydney system defines chronic gastritis as clusters of >5 mononuclear cells in the lamina propria. | 根据更新的悉尼系统改编的一个常用标准将慢性胃炎定义为固有层中>5个单个核细胞簇。 |
Three or more clusters of 6 to 8 plasma cells within the lamina propria is another commonly used criterion. Most pathologists use a more qualitative approach by evaluating the biopsy at low magnification and diagnose chronic gastritis when there is an increase in inflammatory cells within the lamina propria. | 另一个常用的标准是固有层内有6~8个浆细胞形成3个或更多的细胞簇。大多数病理医生使用一种更定性的方法,在低倍镜下评估活检,在固有层内炎症细胞增多时诊断为慢性胃炎。 |
(二)Role of Immunohistochemistry in the Evaluation of Autoimmune Metaplastic Atrophic Gastritis | (二)免疫组化在自身免疫性化生性萎缩性胃炎诊断中的作用 |
Autoimmune metaplastic atrophic gastritis(AMAG) is a disorder characterized by autoantibody-mediated destruction of parietal cells. It is disorder of older women and is the leading cause of pernicious anemia. It has been associated with an increased risk of gastric malignancy. Understanding the pathogenesis of AMAG is crucial for interpreting morphologic and immunohistochemical findings and distinguishing it from other forms of gastritides. Progressive destruction of oxyntic glands has important consequences for the local endocrine environment. With loss of parietal cells, there is decreased production of acid (hypochlorhydria) and intrinsic factor. Decreased gastric acid triggers the antral G cells to upregulate gastrin release which stimulates the parietal cells to increase acid production. This sustained gastrin stimulation has a trophic effect on enterochromaffin-like cells (ECL cells) resulting in ECL cell proliferation and development of NET. | 自身免疫性化生性萎缩性胃炎(AMAG)是一种以自身抗体介导的壁细胞破坏为特征的疾病。它是老年妇女的疾病,是恶性贫血的主要原因。它与患胃恶性肿瘤风险增加有关。了解AMAG的发病机制对于解释形态学和免疫组化结果以及将其与其他形式胃泌素区分开来至关重要。泌酸腺的逐渐破坏对局部内分泌环境有重要影响。随着壁细胞的丢失,酸(胃酸过少)和内因子的产生减少。胃酸的减少触发胃窦G细胞上调胃泌素的释放,刺激壁细胞增加胃酸的产生。这种持续的胃泌素刺激对肠嗜铬样细胞(ECL细胞)有营养作用,导致ECL细胞增生和神经内分泌肿瘤(NET)的发生。 |
Histologically, AMAG produces a corpus-restricted marked chronic inactive gastritis. The inflammation tends to be most prominent in the lower half of the mucosa, around the oxyntic glands. This contrasts sharply with HP gastritis in which the inflammatory activity is localized to the superficial aspect of mucosa. In addition to lymphocytic infiltration and associated gland destruction, many cases also show a prominence of eosinophils. In advanced cases, oxyntic glands may be sparse or entirely absent, and are gradually replaced by pseudopyloric and/or intestinaltype metaplastic glands. | 在组织学上,AMAG胃体局限性慢性非活动性胃炎。炎症倾向于最突出的黏膜下半部分,围绕泌酸腺。这与HP胃炎形成鲜明对比,HP胃炎的炎症活动局限于黏膜表面。除了淋巴细胞浸润和相关的腺体破坏,许多病例还可见明显的嗜酸性粒细胞。在晚期病例中,泌酸腺可能稀疏或完全不存在,并逐渐被假幽门和/或肠型化生腺所取代。 |
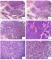
IHC can be useful to support a diagnosis AMAG in two ways: first, AMAG is a disease of the oxyntic mucosa and, therefore, knowing the location of biopsies is important in assessing the type of gastritis. With destruction of oxyntic glands and replacement by pseudopyloric metaplasia, it can be difficult to determine whether the biopsy represents atrophic body/fundus or normal antrum. This is especially problematic when the site of the biopsy is not clearly indicated by the endoscopist or when biopsies from different parts of the stomach are placed in one container. Gastrin IHC can solve this dilemma, as it highlights normal G cells in the antrum (Fig. 1). Pseudopyloric gland metaplasia in the setting of AMAG lacks G cells. Thus, a biopsy fragment that shows paucity of oxyntic glands and lacks G cells on gastric stain confirms that the biopsy fragment originates from the body/fundus. | IHC可以从两个方面支持AMAG的诊断:首先,AMAG是一种泌酸胃黏膜病变,因此,了解活检的位置对于评估胃炎的类型很重要。随着泌酸腺的破坏和假幽门化生的替代,很难确定活检是否代表萎缩的胃体/胃底或正常的胃窦。当内窥镜未明确指出活检部位,或将来自胃不同部位的活检组织放在一个容器中时,这一点尤其有问题。胃泌素IHC可以解决这个难题,因为它明确了胃窦中的正常G细胞(图1)。AMAG背景下的假幽门腺化生缺乏G细胞。因此,一个活检组织块显示缺乏泌酸腺和胃染色上缺乏G细胞,证实活检组织来源于胃体/胃底。 |
The second use of IHC in the setting of AMAG is to highlight ECL cell hyperplasia. The fragments that lack G cells usually show ECL cell hyperplasia and either chromogranin or synaptophysin can be used for this purpose. However, synaptophysin nonspecifically labels zymogen granules of the chief cells, and therefore chromogranin is the stain of choice. In the setting of AMAG, ECL hyperplasia can range from simple hyperplasia to formation of nodules that meet the criteria for a well-differentiated neuroendocrine tumor (WDNET). The classification system proposed by Solcia et al continues to be the reference for categorizing these proliferations (Table 2). However, this classification has not been validated in clinical setting. For all practical purposes, the descriptions that are commonly used in daily practice include linear hyperplasia (at least 2 linear groups of five or more ECL cells in a row), micronodular hyperplasia ( ≥5 ECL cells in the lamina propria bound by a basement membrane), and NET (ECL proliferations that exceed 0.5 mm in greatest dimension and/or present as endoscopically visible lesions). | IHC在AMAG中的第二个用途是强调ECL细胞增生。缺乏G细胞的片段通常表现为ECL细胞增生,嗜铬粒蛋白(CgA)或突触素(Syn)可用于此目的。然而,Syn非特异性地标记了主细胞的酶原颗粒,因此CgA是首选标记物。在AMAG的背景下,ECL增生可以从单纯增生到形成符合高分化神经内分泌肿瘤(WDNET)标准的结节。solcia等人提出的分类系统仍然是对这些增生进行分类的参考(表2)。然而,这一分类尚未在临床上得到证实。实际上,在日常实践中常用的描述包括线性增生(至少两个线性组,每组5个或更多ECL细胞)、微小结节性增生(与基底膜结合的固有层中≥5个ECL细胞)和NET(ECL增生最大尺寸超过0.5mm和/或表现为内镜下可见病变)。 |
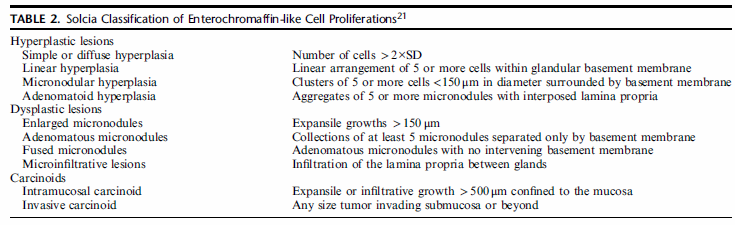 |
表2:肠嗜铬样细胞增殖的Solcia分类 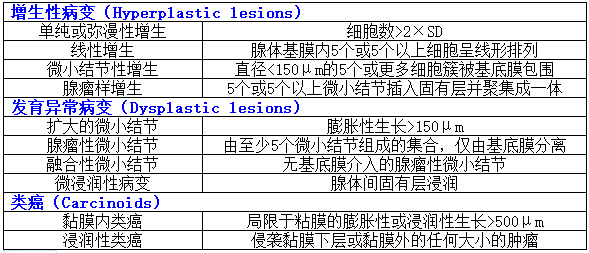
|
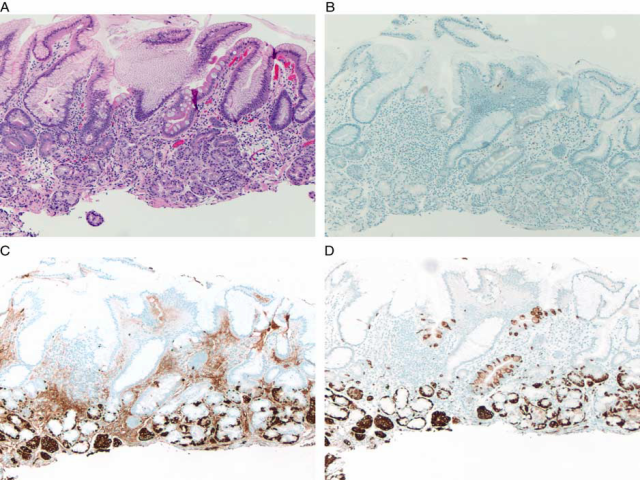 FIGURE 1. Autoimmune atrophic gastritis. A gastric body biopsy from a 62-year-old woman with atrophic appearing gastric corpus mucosa on endoscopy shows chronic inactive gastritis with intestinal metaplasia and paucity of oxyntic glands (A). A negative gastrin stain confirms that the tissue is from the gastric body/fundus (B). Immunostains for chromogranin (C) and synaptophysin (D) demonstrate linear and micronodular enterochromaffin-like cells cell hyperplasia. 图1自身免疫性萎缩性胃炎:患者,女性,62岁,胃镜下胃体黏膜萎缩,胃体活检显示慢性非活动性胃炎伴肠化生和泌酸腺缺乏(A)。胃泌素阴性染色证实组织来自胃体/胃底(B)。CgA(C)和Syn(D)免疫染色显示线性和微小结节性肠嗜铬样细胞增生。 |
















共0条评论